Intertek Blog
The Intertek Blog is a trusted source for insights on product safety, quality, sustainability, innovation, and compliance. The articles written by our experts explore industry trends, emerging technologies, regulatory developments, and best practices, delivering the knowledge and perspective you need to stay informed and inspired.
Most Recent Posts

04 Jun 2025
Understanding Regulatory Frameworks for Single-Use Systems: USP <665>, USP <1665>, and BPOG Extractables Guidance in Biopharmaceutical Manufacturing
What pharma and biopharma manufacturers need to know – and do – before the 2026 deadline.

03 Jun 2025
RED Directive: The Cybersecurity Compliance Countdown – Part 5
Lessons from the Field on Closing Compliance Gaps

02 Jun 2025
Pursuing Transparency: A CDP Climate Disclosure Journey
An overview of how CDP reporting drives climate transparency and sustainable progress


28 May 2025
The Clock is Ticking: Get Ready for the EU's Ecodesign & Digital Product Passport
The EU’s ESPR, effective June 2024, prioritizes textiles for sustainable ecodesign and transparency through the Digital Product Passport. With delegated acts from 2027, the regulation drives circularity, durability, and supply chain collaboration in the textile industry.

23 May 2025
MyPest: U.S. EPA Launches a Pesticide Submission Tracking App
Real-Time Insights for Pesticide Product Registrant
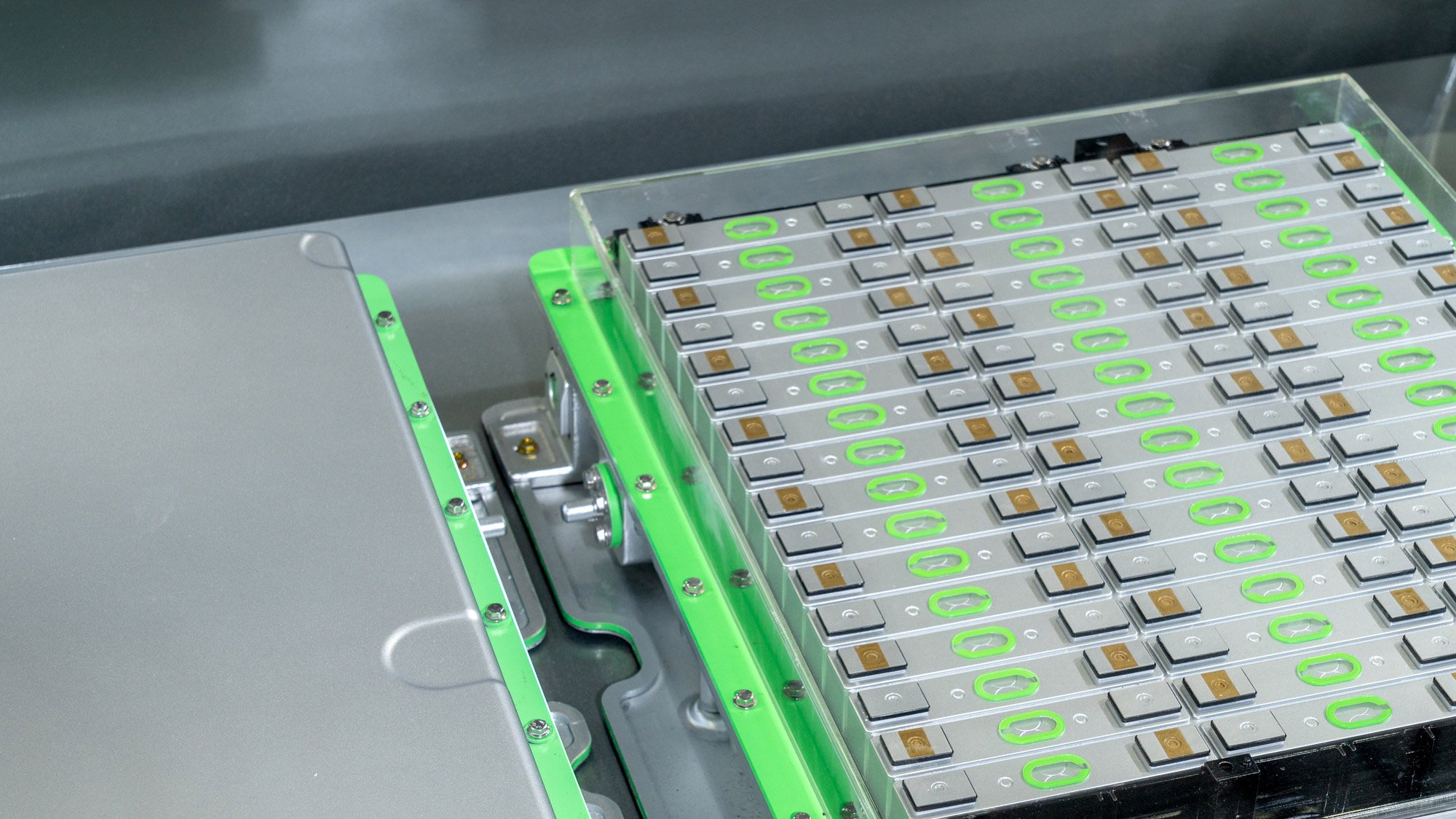
22 May 2025
What does the proposed extension of the battery due diligence deadline for the EU Battery Regulation mean for battery manufacturers and users?
Understand how the EU’s proposed postponement of the battery due diligence obligation deadline will impact your compliance preparations for products regulated by the new EU Battery Regulation, and how you can get ahead.

22 May 2025
Understanding the 2023 Updates to the CSA C22.2 No. 301 Standard – Part 2
An Overview of the Physical and Mechanical Requirements for Industrial Machinery Installations

20 May 2025
RED Directive: The Cybersecurity Compliance Countdown – Part 4
Using Technical Documentation as Your Compliance Safety Net

15 May 2025
Understanding the 2023 Updates to the CSA C22.2 No. 301 Standard – Part 1
A First Look at Key Changes Impacting Manufacturers and Electrical Inspectors under the Revised Industrial Electrical Machinery Standard

13 May 2025
Validating a New Solar Factory in the U.S. from Abroad
What Global Manufacturers Need to Know

09 May 2025
Understanding Biocides and Their Regulation in Canada
Explore the types of biocidal products in Canada and the main regulations that control their approval, marketing, and use nationwide.

08 May 2025
PFAS-Free Future: Insights from the 2025 AATCC PFAS Conference for the Textile Industry
The 2025 AATCC PFAS Conference emphasized the critical need to eliminate PFAS in textiles, showcasing new technologies, regulatory updates, and strategies for manufacturers to achieve a PFAS-free, sustainable future.

06 May 2025
RED Directive: The Cybersecurity Compliance Countdown – Part 3
How EN 18031 Shapes Development of Products that are Secure by Design

05 May 2025
Be reasonable: Increasing Confidence in Companies’ Climate-Related Disclosures (Part 1)
Explore Why Assurance Is the Foundation for Building Trust in Companies' Climate-Related Data and Disclosures