ISO 22716 Cosmetics Good Manufacturing Practice (GMP) compliance auditing solutions including shared audits or private audits to drive product conformity and visibility of your supply chain
ISO 22716 cosmetics GMP quality assurance audits help to drive the quality aspects of production, control, storage and shipment.
Auditing helps to ensure the quality and consistency of your cosmetic products and that they are manufactured according to GMP requirements. ISO 22716 quality audits can also help to identify risks associated with product quality or processes prior to inspections across your cosmetic supply chain. All of these benefits help to your protect brand reputation.
Our specialist GMP cosmetics auditing solutions are delivered by a global network of highly trained professional auditors who have both local knowledge and an in-depth understanding of the relevant national and international regulations. These solutions include shared audit solutions, audits to GMP ISO 22716, training and e-learning tools.
Shared Audits Solutions for the Cosmetics Industry
Cosmetics supply chains are complex and vast. Maintaining your supplier audit schedule may present challenges with respect to ease of scheduling and budgets. As the expectation for supplier or subcontractor audits are forecast to increase, these challenges are set to increase.
To mitigate these challenges our cosmetics auditing team provide a shared audit solution by combining request from multiple cosmetics manufacturers to audit a particular supplier site. Shared audits can reduce demands on time and resources for all stakeholders in the supply chain This in turn can help to optimise your budget through reduced audit costs. The impartiality and independence of our auditors are fundamental to our shared audits solutions and confidentiality is assured throughout the shared audit process.
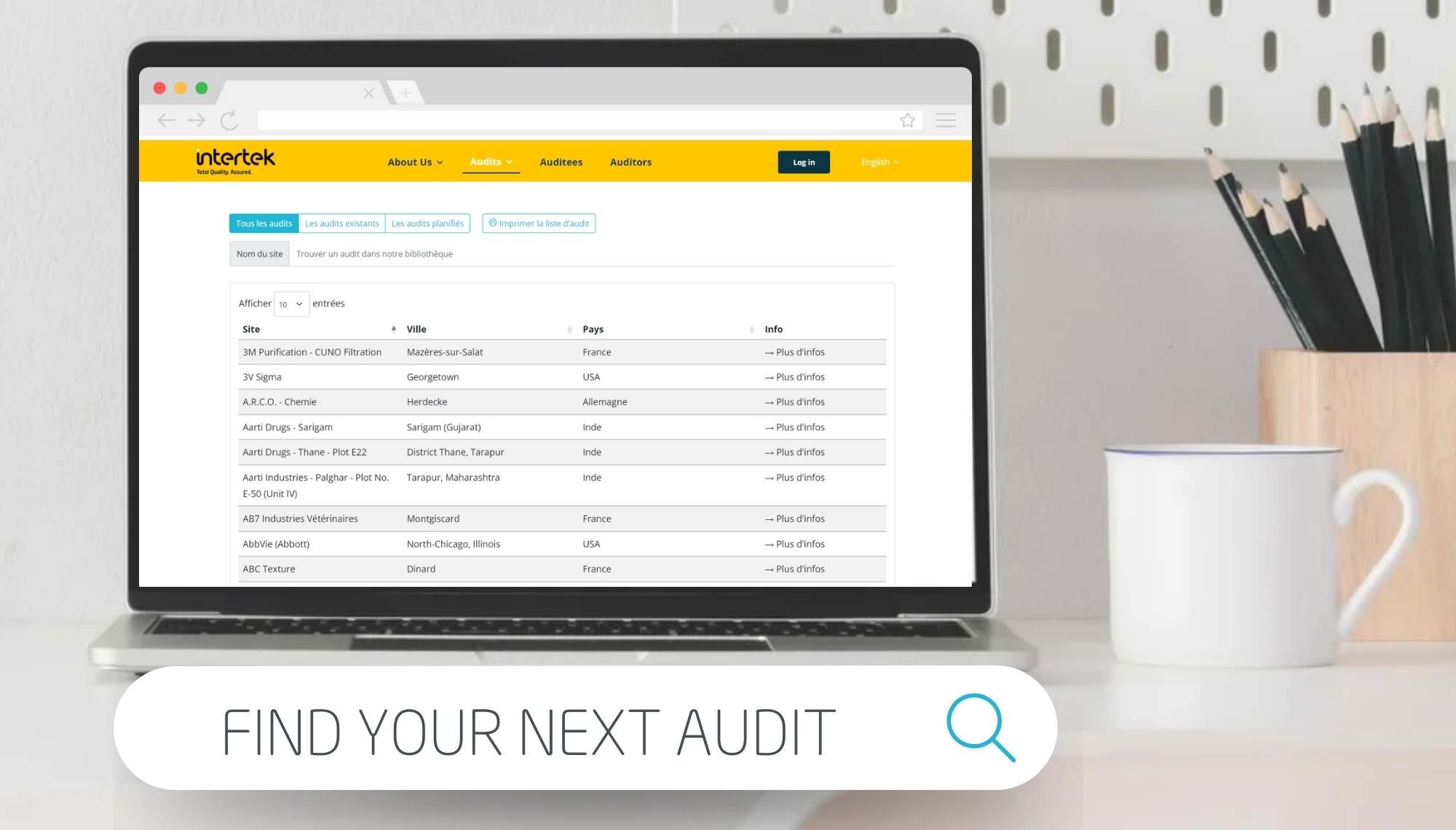
With our new Audit Live Tool, get direct access to our audit reports, join our scheduled audits, or consult our list of over 5000 previously audited sites for your new audit requests.
GMP ISO 22716 Audit Solutions
In Europe, the Regulation (EC) 1223/2009 required cosmetics products to be manufactured according to Good Manufacturing Practices as specified by the ISO 22716 guidelines International Standard which provides guidelines for the production, control, storage and shipment of cosmetic products. The objective of these GMP for cosmetics guidelines is to define the activities that enable you to obtain a product that meets defined quality and safety characteristics through development of quality of assurance and risk assessment.
Private or Shared Audits
Our GMP ISO 22716 audit solutions include pre-audit inspection, training and formal audits and use highly relevant ISO 22716 audit checklists. These services are delivered by our network of BPF auditors and trainers located in Europe, America and Asia. Our auditors have proven experience in the cosmetics sector, helping you to demonstrate GMP compliance and reduce risk of non-compliance. Find out how taking part in our shared audits program can benefit your business with our further resources.
Audit Reports Purchase
In some cases, we are able to offer the purchase of an existing audit report. This a confidential process where you will be able to purchase an existing audit report, done on behalf of another customer. This represents cost-savings to both audit report owner and enquirer and can present a useful alternative to consider if a physical audit is difficult to schedule
Remote Audits
Remote audits are becoming a popular choice to mitigate the challenges posed by travel restrictions. Our global team can provide efficient and comprehensive remote audits for your suppliers worldwide.
Internal Audits
By partnering with Intertek for your internal audit programs, our auditor’s objective approach. and the challenges they highlight help you to drive continuous improvement.
Our Total Quality Assurance Expertise for the Cosmetics Industry
Across our audit solutions, our highly trained and experienced auditors act with total impartiality and independence. They are typically experienced with the local culture and language at the audit location. We are ISO 9001 certified. Quality is at the heart of our organisation and we continuously focus on improving the performance of our services in order exceed expectations of our global clients.
With over 1600 global clients who put their trust in us to meet their auditing requirements, our shared audit system and audit services provide cost-effective and responsive auditing solutions for the global pharmaceutical and healthcare industries. With 30 years’ international experience in auditing and evaluating production and distribution sites, Intertek offers you a truly tailored and unique service that will meet your specific needs. Our Total Quality Assurance expertise enables you to identify and mitigate the intrinsic risk in your operations, supply chains and business processes